How To Find Frequency Factor And Activation Energy
6.two.3.2: The Arrhenius Equation
- Folio ID
- 3818
This folio examines rate abiding variation with temperature and activation free energy, every bit shown by the Arrhenius equation.
Rate Constants and Charge per unit Equations
The rate equation for a reaction between two substances, A and B, is the post-obit:
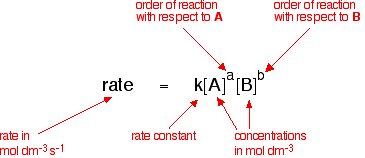
The charge per unit equation shows the effect of changing the reactant concentrations on the rate of the reaction. All other factors affecting the rate—temperature and goad presence, for case—are included in the rate constant, which is only abiding if the only change is in the concentration of the reactants. If the temperature is inverse or a catalyst is added, for instance, the charge per unit constant changes. This is shown mathematically in the Arrhenius equation:
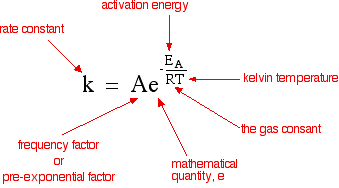
The diverse symbols stand for the following:
- Temperature, T, measured in Kelvin.
- The gas constant, R: This is a constant which comes from the ideal gas law, \(PV=nRT\), which relates the pressure, volume and temperature of a particular number of moles of gas.
- Activation energy, EA : This is the minimum free energy needed for the reaction to occur, expressed in joules per mole.
- due east: This is a mathematical constant with an estimate value of two.71828.
- The expression, \(due east^{-(E_a/RT)}\): the fraction of the molecules present in a gas which have energies equal to or in backlog of activation energy at a detail temperature.
- The frequency cistron, A: Also known as the pre-exponential gene or the steric factor, A is a term which includes factors similar the frequency of collisions and their orientation. It varies slightly with temperature, although not much. Information technology is often considered constant beyond small-scale temperature ranges.
The Arrhenius equation oft takes this alternate grade, generated by taking the natural logarithm of the standard equation:
\[ \big \ln k = \ln A - \dfrac{E_a}{RT}\]
Temperature Effects on Chemic Kinetics
The Arrhenius equation tin can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction. If the rate constant doubles, for example, then does the charge per unit of the reaction.
Example \(\PageIndex{one}\):
What is the kinetic effect of increasing temperature from twenty°C to 30°C (293 K to 303 Thou)?
Solution
The frequency gene, A, is approximately abiding for such a small temperature modify. This problem concerns the quantity east-(Due eastA / RT), the fraction of molecules with energies equal to or in excess of the activation energy.
Let's assume an activation free energy of l kJ mol-ane, or, equivalently, J mol-one. The value of the gas constant, R, is 8.31 J K-ane mol-ane.
At xx °C (293 Thousand) the value of the fraction is:
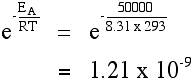
Raising the temperature (to 303 Thou) increases the quantity:
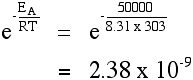
Yous can see that the fraction of the molecules able to react has virtually doubled past increasing the temperature by ten°C. The charge per unit of reaction is about doubled.
The Consequence of a Catalyst
A goad provides a reaction route with a lower activation energy. Suppose that the catalyzed activation energy is to 25 kJ mol-1. The calculation is repeated at 293 Grand:
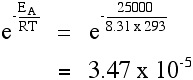
Compared with the corresponding value for an activation energy of fifty kJ mol-1, there is a significant increment in the fraction of molecules able to react. There are almost thirty,000 times more than molecules which tin can react in the presence of the catalyst compared to having no catalyst (using our assumptions about the activation energies). These calculations tin also be washed in reverse: given the rate of reaction or rate constants at several temperatures, the activation energy can be calculated.
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_%28Physical_and_Theoretical_Chemistry%29/Kinetics/06:_Modeling_Reaction_Kinetics/6.02:_Temperature_Dependence_of_Reaction_Rates/6.2.03:_The_Arrhenius_Law/6.2.3.02:_The_Arrhenius_Equation
Posted by: slaytonopeashom.blogspot.com

0 Response to "How To Find Frequency Factor And Activation Energy"
Post a Comment